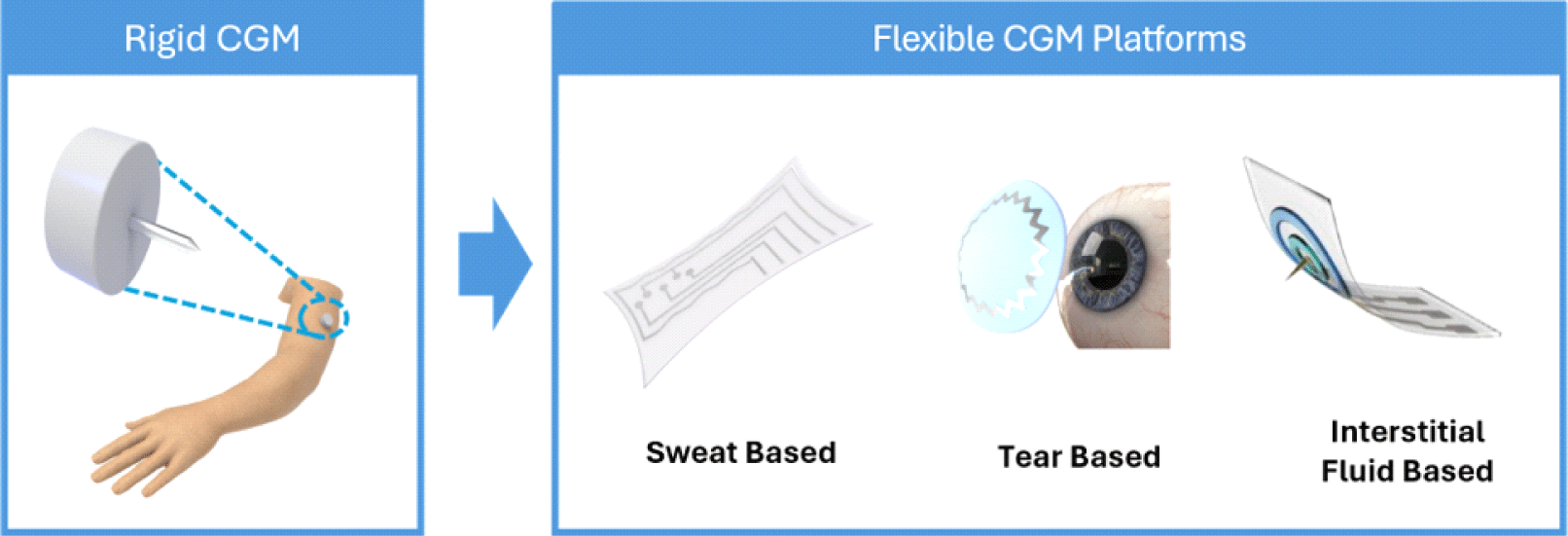
1. INTRODUCTION
Continuous glucose monitoring (CGM) systems have revolutionized diabetes management over the past two decades by enabling continuous, real-time glucose monitoring [1,4]. CGM systems currently available in the market comprise sensors, transmitters, receivers, and compatible smart devices. The sensors measure glucose levels by inserting flexible filament needles into the skin. The transmitter then wirelessly sends glucose data to the receiver, significantly improving the patient’s life from painful traditional fingerstick testing along with tracking of real-time glucose fluctuations.
The primary operational mechanism in CGMs, including those from leading brands like Dexcom, Abbott, and Medtronic, which hold the majority of the global CGM market share, is electrochemistry [5]. While there are devices that employ different mechanisms such as optical biosensors, a significant proportion of both commercially available and prototype CGMs rely on electrochemical sensors due to their high sensitivity and accuracy in measuring glucose levels. These sensors function by utilizing enzymatic reactions, where glucose oxidase (GOx) or glucose dehydrogenase (GDH) catalyzes the oxidation of glucose, generating an electrical signal proportional to the glucose concentration. The sensors then detect reaction byproducts, such as hydrogen peroxide or reduced cofactors, to quantify glucose levels [1-3,6-9].
The global CGM market is expected to exceed $21 billion in market size by 2029 and $11.63 billion in 2024, with a Compound Annual Growth Rate (CAGR) of approximately 12.89% [5]. This impressive growth rate highlights the substantial commercial success of CGM systems, instilling confidence in their potential impact on diabetes management.
However, despite their benefits compared to traditional systems, commercially available CGM devices have limitations that cannot be ignored due to their rigid and invasive nature. Although the level of pain is reduced compared with traditional methods, CGM devices are not completely pain-free. CGM devices on the market have a wearable time of 7 to 14 days, which requires weekly or biweekly replacement of the sensors [10,13]. In addition, due to the inflexible properties of these sensors, strong adhesives or extra over-tapes are used to secure the devices on areas such as the upper arm or abdomen to prevent displacement and ensure stable and continuous glucose sensing. Skin irritation and allergies can occur due to these adhesives, resulting in an uncomfortable user experience [14,19]. Therefore, the need for non-invasive and pain-free glucose monitoring sensors that can overcome these limitations is high, emphasizing the importance of developing flexible CGM devices. Unlike rigid CGMs, flexible CGMs use soft and pliable materials that conform to the body’s contours, enhancing comfort and more natural movement.
Since the advent of flexible electrodes, substantial progress has been made in the field of wearable electrochemical sensors. Numerous prototypes have been developed to detect biomarkers in biofluids beyond blood, including sweat, tears, and interstitial fluid, in a minimally invasive or non-invasive manner [1,3]. These prototypes are designed to accommodate the unique properties of various biofluids with the ultimate goal of achieving seamless integration with diverse surfaces and biological systems.
However, the availability of minimally invasive or non-invasive flexible CGM sensors in the marketplace remains limited, with several key areas still needing to be addressed [1,2,20]. Biofouling continues to be a significant limitation to sensor stability and precision. Technical challenges such as noise from human motion and interference from pH, temperature, and fluid conductivity must be systematically addressed to ensure data accuracy.
On the clinical side, achieving accuracy comparable to traditional fingerstick methodologies and commercialized CGMs remains a challenge for flexible CGMs using different biofluids. Establishing reliable correlations between various biofluids and blood glucose levels is critical for clinical acceptance. While ISF has shown high relevance, biofluids such as sweat, and tears still require further validation for commercialization. Flexible CGMs need to undergo stringent clinical validation to ensure they provide reliable data for medical decision-making.
Recent studies have made significant progress in addressing these challenges through innovative sensor design, materials, and fabrication techniques. These advancements are not merely theoretical possibilities but tangible solutions, expected to foster further innovations and accelerate the commercialization timeline of flexible CGMs in the market.
This review examined the most recent developments in flexible CGM devices. Initially, we present an overview of current rigid CGM sensors and their limitations, addressing specific challenges. Subsequently, we introduce the latest advancements in flexible CGM prototypes developed in the past three years, based on different biofluids and sensor platforms. These include epidermal sensors for sweat measurement, smart lenses for glucose sensing in tears, and monitoring interstitial fluid (ISF) glucose using flexible microneedle sensors. Finally, we discuss the perspectives and prospects of flexible CGM devices, taking a comprehensive approach for successful commercialization in the near future.
2. RIGID CGM SYSTEMS
CGM systems have significantly impacted diabetes trends, facilitating optimal glycemic control. Compared with the traditional blood glucose monitoring method which requires multiple fingerstick per day, CGMs have offered less painful ways to measure glucose levels. The latest dominant models on the global market include Dexcom G7, Freestyle Libre 3 by Abbott, Guardian 4 by Medtronic, and Eversense E3 by Senseonics [10,13] (Table 1).
| Company | Dexcom | Abbott | Medtronic | Senseonics |
|---|---|---|---|---|
| Model | G7 | Freestlye Libre 3 | Guardian 4 | Eversense E3 |
| Wear time | 10.5 days | 14 days | 7 days | 180 days |
| Platform | Patch type/invasive | Patch type/invasive | Patch type/invasive | Implant type/invasive |
| Position and insertion | Arm or abdomen/self-inserted | Upper arm/self-inserted | Arm or abdomen/self-inserted | Arm/inserted by healthcare provider |
| Monitoring mechanism | Electrochemistry | Electrochemistry | Electrochemistry | Optical-fluorometry |
| Website | https://www.dexcom.com/ | https://www.freestyle.abbott/us-en/ | https://www.medtronicdiabetes.com/ | https://global.eversensediabetes.com/ |
The Dexcom G Series, Freestyle Libre, and Guardian models are patch-based systems that utilize subcutaneous electrochemical sensors for glucose measurement in the ISF. The Dexcom G Series employs a glucose oxidase-based sensor that produces an electrical current proportional to the glucose concentration and transmits data to a receiver or smartphone application for continuous monitoring and alerting of glycemic events. The Freestyle Libre and Guardian models also use electrochemical sensors to provide real-time glucose monitoring and predictive alerts.
These sensors can be self-applied by pressing an applicator that has a needle attached to punch the flexible filament sensor into the skin. The needle is retracted once the sensor is implemented.
On the other hand, Eversense by Senseonics features a longer-term implantable sensor with a different mechanism. Unlike other patchable CGM models, Eversense measures glucose levels by detecting changes in the fluorescence emitted by a glucose-responsive polymer implanted under the skin for up to 180 days [21]. The chargeable transmitter is worn externally over the sensor insertion site with an adhesive patch. The insertion and removal process takes several minutes, which is conducted by the health care provider.
Incision of approximately 5 mm to 1 cm in the upper arm is made after using local anesthesia to numb the skin. After the sensor is placed, the strip is applied over the incision area as a cover [22].
Although the current CGM system offers numerous benefits, they also present critical limitations, causing skin irritations such as allergic contact dermatitis (Fig. 1). Skin adverse effects are the most common reason for discontinuation of CGM [15]. The inflexible sensor materials used in patch-based systems can cause discomfort, particularly during prolonged use. Inflexible devices are more prone to fracture and detachment when subjected to the natural stretching and contracting of the body, as they cannot form conformable contact with the curvilinear human skin. Therefore, these wearables are usually constrained to specific body locations, thus limiting the wearers’ mobility [16,18,19]. The mechanical mismatch between these rigid sensors and the soft, dynamic nature of the human skin can result in sensor displacement, requiring a strong adhesive or additional patch application to hold rigid sensors from falling off. Isobornyl acrylate (IBOA), a common component of these adhesives is a known allergen that can lead to significant skin irritation [14].
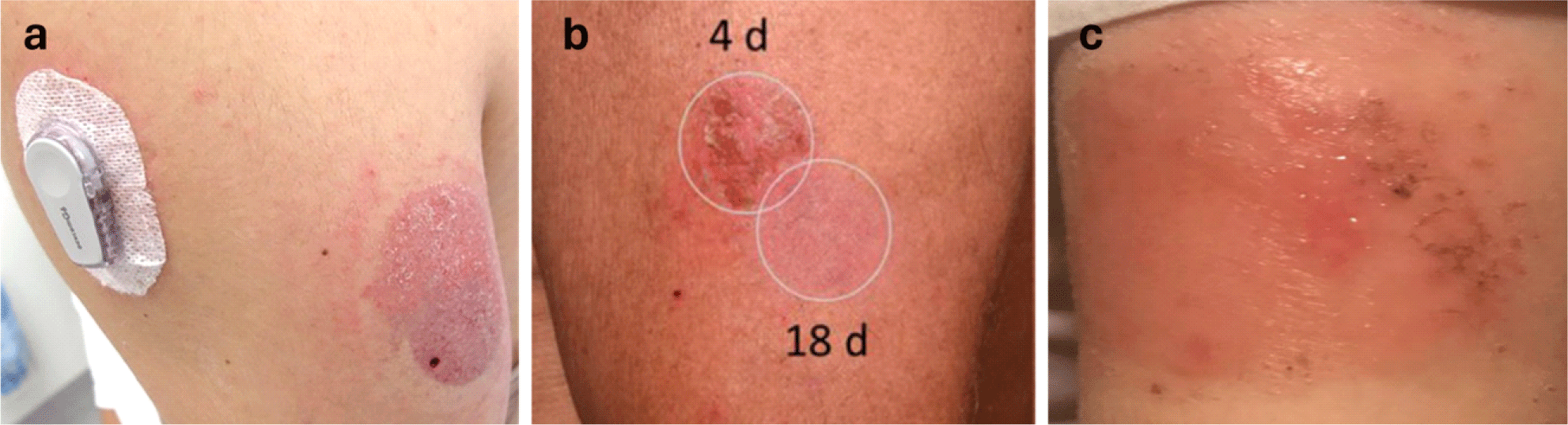
In addition, the duration of the devices is 7−14 days, requiring weekly or biweekly replacement. Repetitive occlusion, friction, and increased humidity underneath adhesives can disrupt the skin barrier, leading to irritant contact dermatitis. A damaged skin barrier becomes more permeable to allergens contained in the adhesives, subsequently increasing the risk of allergic contact dermatitis [15]. Unannounced modifications in adhesive compositions exacerbate these issues, leading to unanticipated skin reactions. The emergence of new allergens from adhesive formula changes, such as 2,2’-methylenebis (6-tert-butyl-4-methylphenol) monoacrylate, complicates the clinical management of patients sensitized to multiple allergens [17]. Furthermore, the CGMs may cause bruising or bleeding from the needle inside the applicator. Although the risk is low, few incidents of sensor wire breaking under the skin has been reported [23].
The implantable type CGM requires a minor, yet still surgical procedure for sensor insertion and removal. These invasive processes present additional challenges including the risk of site infection or inflammation. Therefore, developing sensors with great skin conformality using biocompatible materials is essential to address the limitations of existing rigid CGM devices.
3. FLEXIBLE CGM DEVELOPMENT
Sweat is a biofluid produced by the eccrine sweat glands in the dermis layer of the skin. It can be easily obtained and abundant in nature, making it a desirable option for non-invasive biosensors [2]. Although the amount of glucose in sweat is relatively minor compared to blood or ISF, which are also used in conventional glucose sensors, studies have demonstrated that sweat glucose can effectively reflect blood glucose levels if collected correctly and safeguarded from contamination [1]. Therefore, a reliable sweat-based CGM that is stable under various environmental conditions, such as changes in temperature, humidity, and physical activity must be developed. Furthermore, the importance of maintaining intimate contact for precise physiological monitoring is especially emphasized in epidermal sweat-based sensors, as sweat is gathered and analyzed on the skin’s surface.
Over the past decade, various sweat-based flexible CGM prototypes have been developed and categorized into two types based on the sweat sampling method [1,2]. The first is patch-based sensors that directly interface with the skin, which generally have more straightforward features and are sometimes integrated with additional features, including drug delivery systems [24]. The second is a microfluidics-based sensor that channels sweat to the sensors to minimize contamination and ensure accurate readings [25]. Studies on both types are ongoing, and several notable advancements have been made. These enhancements include efficient integration of sweat stimulation and analysis, as well as improvement of manufacturing compatibility.
In 2022, Wang and Sempionatto’s group introduced an epidermal platform that could stimulate, collect, and analyze glucose levels in sweat using a single integrated device (Fig. 2(a)) [25]. The new device is the first prototype to integrate sweat stimulation through the transdermal delivery of pilocarpine using the iontophoresis process in a microfluidic CGM using a skin conformal elastomeric polydimethylsiloxane (PDMS) with soft lithography and screen-printed sensors. Previous research methods typically required intense physical exercise to induce sweat secretion, greatly affecting user convenience [1,2,24]. On the other hand, iontophoresis can deliver pilocarpine into the skin to stimulate sweat glands and promote sweat secretion at the sensor site in a much easier, quicker, and more controlled manner with less interference from body movements.
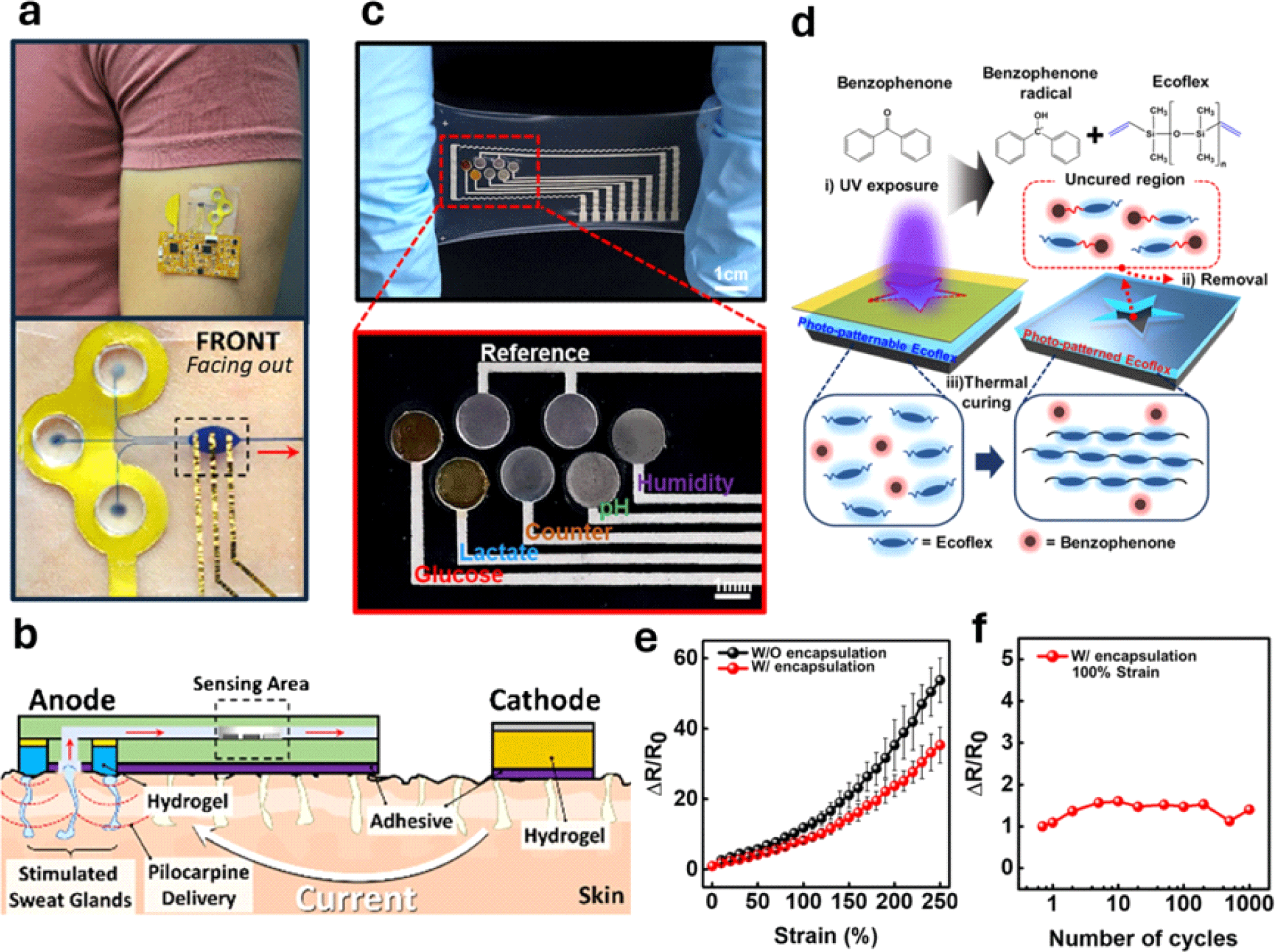
This innovative approach also increases the accuracy and reliability of continuous glucose monitoring by overcoming one of the major challenges of analyte dilution. It enables constant supply of fresh sweat to the sensor, free from mixing old sweat, making it suitable for prolonged use.
For iontophoresis, pilocarpine was mixed into an agarose gel and placed on the anode of the device. When current is applied, electrostatic repulsion drives pilocarpine into the skin. The team optimized the iontophoresis process to maintain a current density of 0.4 mA cm−2 for 10 minutes to ensure efficient sweat generation. The generated sweat was then pumped into the fluidic inlets of the device, flowed through a microchannel made of PDMS, and filled into the oval-shaped reservoir where the electrochemical glucose sensor was located (Fig. 2(b)). Additionally, to prevent cross-contamination with the iontophoretic gel, a pillar-like PDMS layer was developed to physically separate the gels from the sweat collection area, ensuring that only fresh sweat reached the sensor. Moreover, the device was equipped with real-time wireless data transmission through a flexible electronic Printed Circuit Board (PCB) that conforms to the body. The sensor’s performance strongly correlated with the results measured by a commercial glucometer before and after meals [25].
Other recent advancements have been made in terms of the material and process compatibility of the sensor components. Many flexible sensor materials face challenges in being fully compatible with current industrial manufacturing processes, which makes it hard to incorporate these materials into mass production lines [20]. In 2023, Lee and Bae’s group introduced the use of a photopatternable Ecoflex (PPE) in the encapsulation layer of a multi-biochemical sensor prototype that can continuously monitor glucose, lactate, pH, and humidity in sweat, developing a systematic aligning method for patterning stretchable biosensors [26] (Fig. 2(c)). When developing a flexible biosensor, an encapsulation layer holds significant importance in securing longevity and sensitivity, as it plays a crucial role in safeguarding circuit components against degradation and oxidization and acts as an adhesive to the skin surface.
Photolithography has often been used to pattern the encapsulation layer of various organic polymers, such as SU8 and parylene, in flexible electronics. Patterning methods such as soft lithography, punching, and laser ablation have been typically used to pattern soft and stretchable materials [27,29]. However, they often require additional steps such as lamination, which is time consuming with low yield. Although a photopatternable method has been demonstrated on PDMS in previous studies, it was not used for Ecoflex because of its short pot life [30]. Despite its high stretchability and good adhesive strength, Ecoflex cures rapidly, even at room temperature, making the photopatterning process challenging.
To tackle this issue, the team modified Ecoflex to be photopatternable by adding benzophenone, which acts as a curing retarder and photo-inhibitor, allowing sophisticated patterning directly on the material. When UV light shines on benzophenone, it induces free radicals, which prevent Ecoflex from hardening as they react with it. In the parts of Ecoflex that are exposed to UV light, the free radicals keep it soft, while the unexposed parts harden during a heating step known as a pre-bake (Fig. 2(d)). Benzophenone absorbs light most effectively at 260 nm, but can still react up to 365 nm, allowing the process to work with light below 365 nm. In addition, benzophenone radicals are not affected by atmospheric oxygen, indicating that PPE can be patterned in normal air without requiring a special environment.
To test the effect of the PPE encapsulation layer on the electrical properties of the stretchable conductor, the group conducted a strain test on the encapsulated Ag flake, which showed stable electrical characteristics even when stretched up to 250% strain. The encapsulated electrode demonstrated a 50% increase in electrical conductance under 250% strain, highlighting the effectiveness of the encapsulation layer in maintaining the performance under mechanical stress (Fig. 2(e)).
In addition, the conductor retained its electrical characteristics after 1,000 cycles of repetitive deformation at 100% strain, whereas the performance of the non-encapsulated conductor was maintained under similar conditions (Fig. 2(d)). To develop a patchable sensor prototype, the team deposited gold nanoparticles (AuNPs) on Ag flakes through a galvanic replacement process to make it more biocompatible and resistant to oxidation. The stretchable sweat sensor continuously monitored glucose levels in real time, providing immediate feedback on glucose concentration changes [26].
Tears have the characteristics of easy accessibility and continuous flow, which allows for the opportunity for non-invasive glucose monitoring. Glucose in tears is attributed to “plasma leakage,” in which blood components partially leak into tear fluids through a barrier. This makes it feasible to utilize tear fluid as a proxy for analyzing blood composition. By leveraging the correlation between blood and tear glucose, as well as sodium (Na+), potassium (K+), and chloride (Cl−) ion concentrations, contact lens sensors can serve as continuous and minimally invasive diagnostic instruments [31]. It is important to comprehend the mechanisms and characteristics of tears to achieve accurate readings as there are three types of tears with distinct attributes: basal, reflex, and psychic tears. While basal tears containing glucose are constantly produced, the other two types of tears are not released continuously and have different compositions. Reflex tears are triggered by physical and chemical stimuli in the eye to eliminate foreign substances and safeguard the eye. Psychic tears are associated with emotional stimuli such as anger or pleasure and possess significantly higher concentrations of hormones. Therefore, tear-based sensors must be designed to prevent irritation in order to minimize the occurrence of reflexes and psychic tears.
Conventionally, physical methods have been used to analyze tear drops, including tear film breakup time, osmolarity, tear volume, viscosity, and surface tension. Traditional tear collection methods include Schirmer strips, capillary tubes, and surgical sponges [32]. However, these methods are directly applied to the eye, often causing irritation that can induce reflex tears, allowing only one-time-point measurements, and causing discomfort for patients. Additionally, collection of a large sample volume can lead to inaccurate concentration measurements. Thus, caution is needed when considering the concentration ranges, which may be affected by the sampling method.
Owing to the introduction of smart contact lenses (SCLs), a simpler and more patient-friendly approach to tear component detection has become feasible. Due to their non-invasive nature, SCLs have advantages over conventional methods. They are worn and rested directly on the cornea, offering greater comfort than conventional invasive methods, while enabling continuous monitoring of various physiological parameters. In 2014, Google and Novartis attempted to commercialize SCLs for patients with diabetes, recognizing the potential of this platform (Fig. 3(a)). The prototype sensor included a chip and antenna for wireless communication, a tear glucose sensor, and a soft contact lens [31]. The system utilized inductive coupling and a Li-battery power system [33].
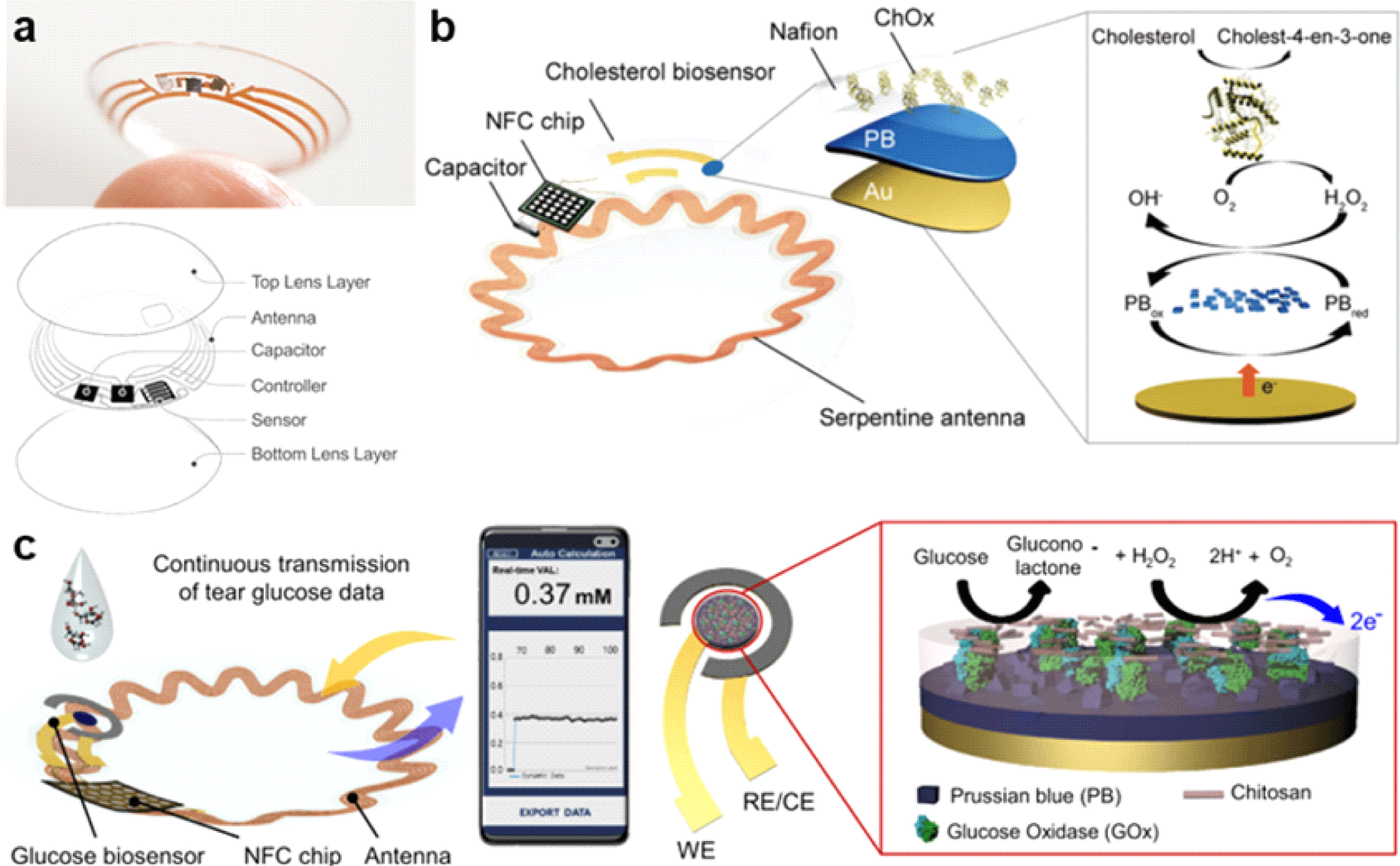
However, the project was discontinued in 2018 owing to the lack of correlation between tears and blood glucose levels required for clinical application. Tear fluid contains a much lower glucose concentration than blood, thus achieving the accuracy of tear-based monitoring has been challenging. In addition, low sensor sensitivity is a major obstacle in clinical applications [34].
In 2022, Park’s group developed a soft and stretchable SCL platform for wireless electrochemical measurement of free cholesterol in tears, enabling continuous real-time monitoring (Fig. 3(b)). The SCL consists of a Near Field Communication (NFC) wireless system with a capacitor, stretchable antenna, and NFC chip embedded into a soft wearable contact lens. The NFC chip includes a current-to-digital converter and digital-to-analog converter connected to a cholesterol biosensor. By using the NFC system, the device communicates with and is wirelessly powered by a smartphone. For wireless sensors, stability against mechanical deformations such as bending and stretching is a prerequisite to minimize inductance changes.
The serpentine antenna design of this device exhibited excellent stability with minimal resistance change when bent to a radius as small as 1 mm, making it suitable for integration into contact lens, given that this curvature is smaller than the radius of curvature of the human eye. Furthermore, the antenna displayed strong resistance attributes over 1,000 cycles of repetitive elongation, reaching tensile strains of up to 30%, which emphasizes its suitability for integration into SCL platform. The cholesterol biosensor measured free cholesterol using cholesterol oxidase, generating H2O2 when it is in contact with free cholesterol. Prussian blue was used on the working electrode to reduce the generated H2O2. Chronoamperometry was used as the measurement method. The developed platform has the potential to be used with various oxidizable compounds such as glucose, lactic acid, and uric acid [35]. Stability was evaluated for a week, and the constant of sensibility was demonstrated.
Another advancement in improving the accuracy of tear-based CGM was introduced in 2024. An important breakthrough in validating the credibility of the correlation between tear and blood glucose levels was achieved by Park’s group. The concept of ‘personalized lag time’ was established and incorporated into the wireless SCL platform previously developed by the team as a crucial element for precisely estimating blood glucose levels. This advancement overcame the challenge that had prominently affected earlier iterations of SCLs, ultimately leading to the cessation of their development [36]. This concept refers to the time delay between changes in blood glucose levels and their corresponding reflections in tear glucose levels.
To fabricate this SCL, a serpentine antenna was created using spin coating and photolithography. The antenna featured a 25 μm-thick PI film substrate made by spin coating. The pattern and contact pad were produced using a photolithography procedure with Cr/Cu and Cr/Au, respectively. The capacitor size was adjusted to 82 pF to enable 13.6 MHz NFC communication. The capacitor and antenna were connected to the NFC chip by wire bonding with gold wire. Additionally, the NFC chip was polished to a 200 μm-thick to fit the SCL system. All components were integrated by molding with silicone elastomer and cured at 70°C under a pressure of 131 kPa for 5 hours. The fabricated SCL was tested in both animals and human subjects to identify the correlation between tears and blood glucose levels. By integrating personalized lag time into their analysis, researchers improved the accuracy of blood glucose level estimation using tear glucose data. This personalization approach enables the achievement of more clinically relevant results and allows technology to be customized for individual users [36].
Interstitial fluid (ISF) has been used extensively as an alternative biological fluid for non-invasive glucose sensors owing to its high similarities in blood biomarker levels [1,4]. The correlation between glucose levels is due to the high permeability of capillary endothelial cells. This thin, semipermeable membrane allows glucose to move rapidly from the blood to the ISF, resulting in nearly identical glucose levels in both compartments. While ISF extraction techniques have mainly been used in early glucose sensing, microneedle (MN)-based ISF sensing strategies have recently gained considerable attention [37]. A microneedle is a very thin needle with a microscopic structure less than 1 mm in length and diameter ranging from a few micrometers to several hundred micrometers. Various materials, including silicon, polymers, and dissolvable substances, are used to fabricate microneedles with different configurations, depending on the intended use [38,39].
There are two main approaches for glucose monitoring using MN-based sensors. The first is indirect sensing, which uses hollow MNs to extract ISF from epidermal layers to conduct sensing on the skin surface. Second, direct on-the-tip electrochemical sensing, where the working electrode of the MN is coated with enzymes or other catalysts such as glucose oxidase (GOx), reacts with glucose in ISF, producing an electrical signal proportional to the glucose concentration [37]. The ISF extraction approach has a few obstacles that must be addressed, particularly in prolonged monitoring. One of the significant issues is the clogging of MNs, which impedes continuous monitoring and necessitates frequent maintenance or replacement of sensors [1]. This challenge has driven a shift in focus towards developing direct on-the-tip sensing technologies, which offer the potential for more reliable and continuous glucose monitoring without the complications associated with extraction issues.
To develop flexible direct on-the-tip sensors, MNs must exhibit sufficient hardness to penetrate biological tissue while maintaining sufficient elasticity to prevent breakage upon insertion into the skin [40]. Concurrently, the substrate must be sufficiently flexible to conform to the dynamic surface of the skin, which is crucial for ensuring continuous and accurate glucose monitoring. However, producing MNs with a high Young’s modulus on a selected area of a conformable submicron-thick substrate can be challenging. A submicron-thick substrate can create high mechanical stress owing to wrinkling and bending, potentially damaging the connection between the needle and the substrate. To mitigate this, materials such as PDMS, which has a low Young’s modulus, can be used [38]. However, PDMS typically exhibits a low surface energy, posing another challenge for durability and adhesion when integrated into flexible MNs.
Research conducted by Lee’s group in 2021 marked a significant advancement in MN sensor platforms. The group successfully integrated two siloxane-based polymers with different stiffnesses to create a 5X5 MN pH sensor array [40] (Fig. 4(a)). To create the sensor array, MNs were fabricated using an epoxy siloxane polymer, which was then placed between a 15 μm thick PDMS substrate and a passivation layer that was bonded using plasma treatment. The strong bonds formed between the MNs, and substrate were due to both dangling effects and covalent bonds, effectively solving the adhesion issue. The MNs had a high Young’s modulus of 3 GPa, which enabled them to penetrate human skin, which has Young’s modulus from 0.08 to 1.32 MPa. The PDMS substrate also exhibited a Young’s modulus of 5 MPa, indicating excellent skin conformability.
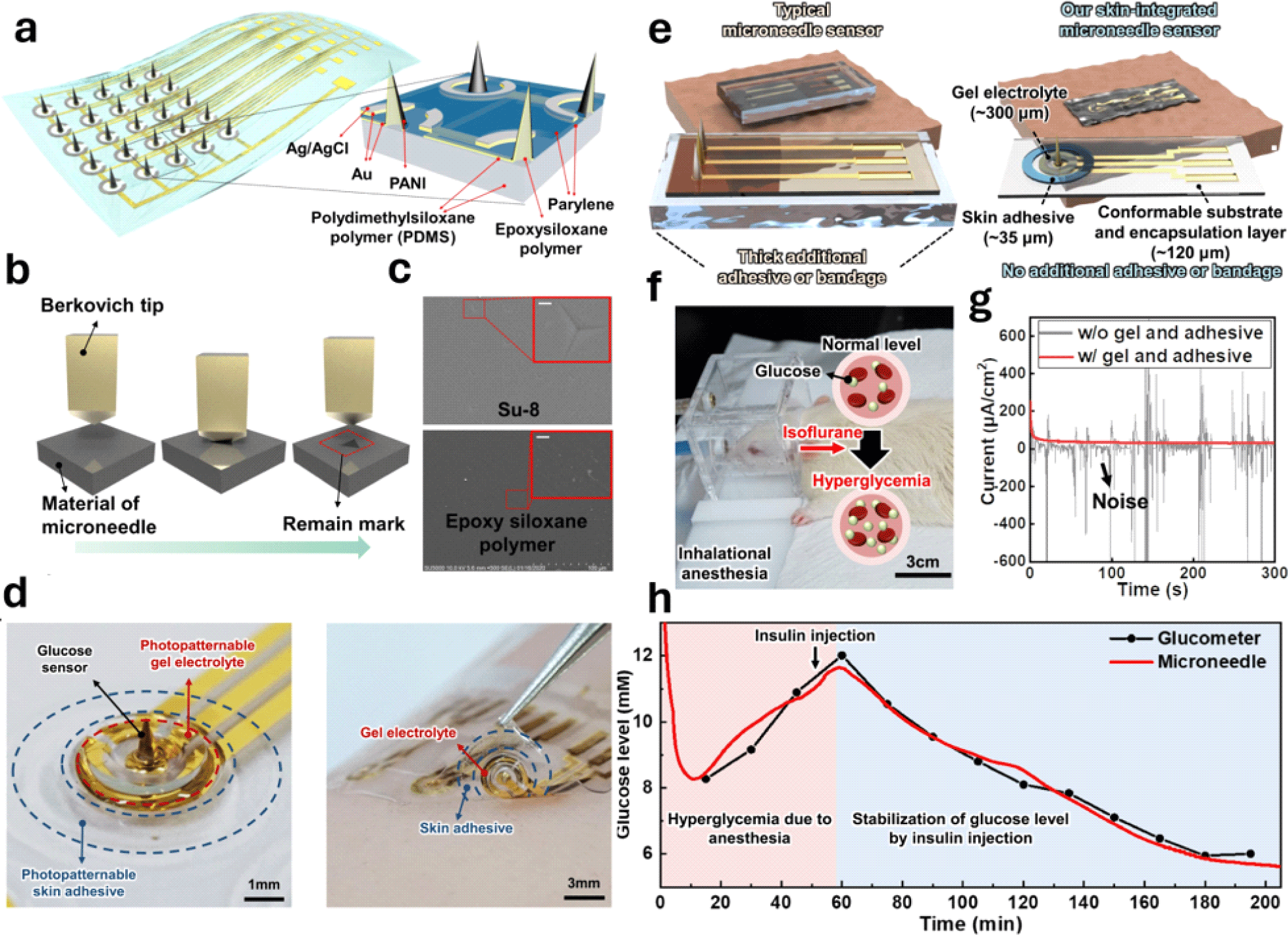
Nanoindentation tests were conducted to measure the hardness and elastic properties of the epoxy siloxane polymer used in the MNs, which helped assess the mechanical stability of the sensor (Fig. 4(b)). This study showed that the epoxy siloxane polymer had better elastic compliance than other materials commonly used in fabricating MNs with high Young’s moduli, such as silicon and SU8, proving its applicability (Fig. 4(c)). The long-term durability of the MN sensors was assessed by repeated insertion into pig skin and bending tests. The results indicated that the sensors demonstrated high pH sensitivity and stability with only minor degradation after multiple uses.
In 2023, Lee’s group further advanced the MN sensor platform in the CGM prototype using epoxy siloxane polymer and PDMS, which were also employed in their 2021 pH prototype [41]. The techniques and materials used for fabricating MNs have been adopted and evolved into enhancing functional soft materials. These materials are essential for achieving stable and accurate measurements of bio-signals by establishing an electrical and physical connection with the skin (Fig. 4(d)).
Existing MN sensor prototypes typically use a thick additional adhesive or pressure bandage, mechanically restricting the skin. However, Lee’s group has developed a skin-integrated MN sensor prototype that weighs only 14 mg and is 150 μm thick, eliminating the need for additional adhesives or bandages (Fig. 4(e)). By utilizing photolithography-based patterning techniques, the team directly patterned the skin adhesive and gel electrolyte onto the device, which is expected to be more efficient and less time-consuming than the traditional methods such as screen printing, spray coating, and ink-jet printing.
The development of this prototype involves several components, including a 60 μm siloxane-based hybrid substrate that is flexible, a 60 μm photopatternable PDMS (PP-PDMS) encapsulation layer, a 35 μm photopatternable skin adhesive (PP-adhesive), and a 300 μm photopatternable gel electrolyte (PP-gel). This setup integrates a flexible PDMS substrate with a Young’s modulus of 5 MPa and MNs made from a photocurable epoxy siloxane polymer with a Young’s modulus of 3 GPa. To reduce invasiveness, only the working electrode out of the three electrodes in the sensor was designed as a needle with a height of less than 1 mm.
This new method not only streamlined the process of manufacturing, but also improved the functionality of these devices. The sensitivity of the MN sensor was assessed using an anesthetized rat to continuously monitor the glucose levels (Fig. 4(f)). Amperometric measurements were conducted on sensors with and without the PP-gel and PP-adhesive. A consistent current response was observed in the sensor that had both PP-gel and PP-adhesive, whereas the sensor that did not have these components showed an unstable response (Fig. 4(g)). Glucose levels were measured using a MN sensor and compared to readings from a commercial glucometer. The results showed stable and accurate glucose measurements that were consistent with glucometer readings. To test the response of the sensor to changes in glucose levels, insulin was injected into the rats. The sensor successfully detected a decrease in glucose levels after insulin injection, indicating its effectiveness for real-time monitoring (Fig. 4(h)).
The stability of the sensor was evaluated through the measurement of its electrical impedance spanning a week, revealing consistent performance. The sensor exhibited strong adhesion to the skin, devoid of any adverse effects such as bleeding or inflammation. Furthermore, time-lapse images displayed no signs of skin irritation following a four-hour application period, proving the safety and reliability of the sensor for prolonged utilization.
Lee’s team accomplished a significant enhancement in manufacturing efficiency by utilizing a singular photolithographic technique to create all the necessary sensor components, including the needle, encapsulation layer, gel electrolyte, and skin adhesive. This makes the device highly suitable for prolonged glucose monitoring, demonstrating its potential for future commercial applications.
4. PERSPECTIVE AND FUTURE PROSPECTS
Advancements in sensor materials and fabrication techniques have opened new avenues for wearable continuous glucose monitoring through various biofluids, such as sweat, tears, and ISF. These innovative, flexible, and biocompatible sensors are designed to provide a non-invasive alternative to traditional fingerstick and rigid CGM systems, which are often associated with skin irritations. As these technologies continue to evolve, they promise enhanced user convenience and reduced pain, marking a significant shift towards more patient-friendly diabetes management solutions.
However, realizing the full potential of flexible CGM sensors requires sustained efforts to overcome persistent challenges before these devices can achieve widespread commercial use. While progress has been made in improving sensor performance, further refinements are necessary to mitigate the impact of external factors that can compromise sensor reliability. Flexible microfluidic systems have shown promise in addressing issues such as analyte dilution, yet advanced optimization and thorough clinical validation are needed to ensure accurate readings during prolonged real-life use.
Collaboration across fields, including materials science and electrical engineering, is essential to ensure precise and reliable wireless data transmission. This involves developing flexible and stretchable materials that maintain consistent resistance levels under strains caused by body movement, which requires a multidisciplinary approach. Additionally, clinical and medical experts play a crucial role by providing insights into the physiological relevance of biomarkers and practical applications of biosensors, ensuring their effectiveness across large populations and in real-world healthcare settings.
Incorporating advanced manufacturing processes to produce flexible sensors on a large scale will be vital for transitioning these devices from prototypes to market-ready products. Moreover, environmental sustainability must be integrated into production and disposal processes, with an emphasis on eco-friendly materials to minimize ecological impacts. As research progresses in these areas, the advancements achieved thus far underscore the considerable promise of flexible CGM sensors. With continuous efforts to address both technological advancements and practical challenges, flexible CGM devices are well-positioned to become the next standard in diabetes care.